南德 TÜV CE证书编号:No.G2S 101395 0003 Rev.00
TÜV SÜD CE certificate:No.G2S 101395 0003 Rev.00
第二类医疗器械注册证:京械注准20202140241
China Class II Medical Device Registration Certificate: 京械注准20202140241
产品分类:无菌、非无菌
Product Classification: Sterile/Non-sterile
适用范围:非无菌产品可用于隔离留观病区(房)、隔离病区(房)医护人员穿的职业防护衣。阻止来自患者的病毒随空气或液体向医务人员传播。
无菌产品适用于隔离重症监护病区(房)医护人员穿的职业防护衣,阻止来自患者的病毒随空气或体液向医务人员传播。
Typical applications:
Medical Protective Coverall-BH800(Non-sterile) is used to provide barrier and protection for the blood, body fluids, secretions, and particles in the air of patients with potential infection that the clinical medical staff come into contact with at work.
Medical Protective Coverall-BH800(Sterile)is used to provide barrier and protection for the blood, body fluids, secretions, and particles in the air of patients with potential infection that the intensive care unit staff come into contact with at work.
一次性医用防护服-BH800
Medical Protective Coverall BH800



一次性医用防护服-产品性能
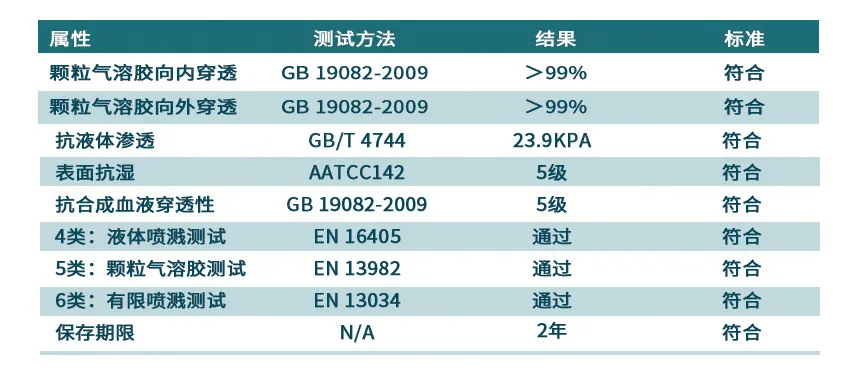
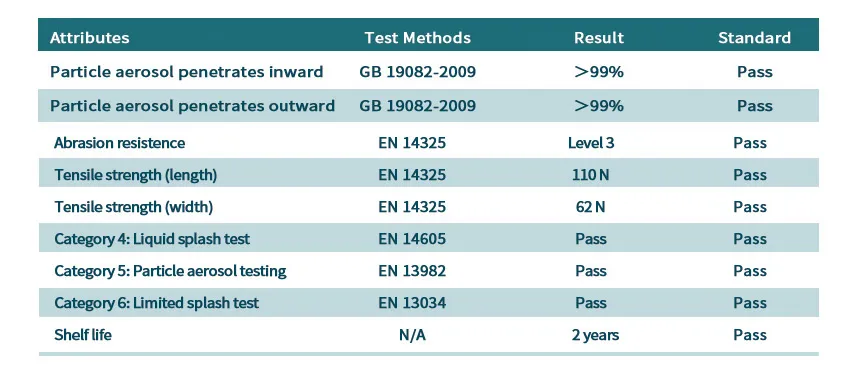
防护性能:外覆防护服专用热封胶带,有效阻隔病毒、细菌、有害超细粉尘、体液血液喷溅。
防静电性能:材质经过抗静电处理,减少有害物质吸附。
Main material: Composite nonwoven fabric of 78g/㎡ is used as raw material, and it is made by cutting and sewing one or more fabrics that can isolate virus aerosols and virus-containing liquids.
Protection performance: Seams are covered with special heat-sealing tape for extra protection. The tape can effectively block viruses, bacteria, harmful ultrafine dust, and body fluids and blood splashes.
Anti-static performance: The material is treated with anti-static treatment, which can effectively reduce the adsorption of harmful substances.
一次性医用防护服BH800-证书明细
ISO 13485证书
ISO 13485 QMS Certificate

CE证
CE Certificate

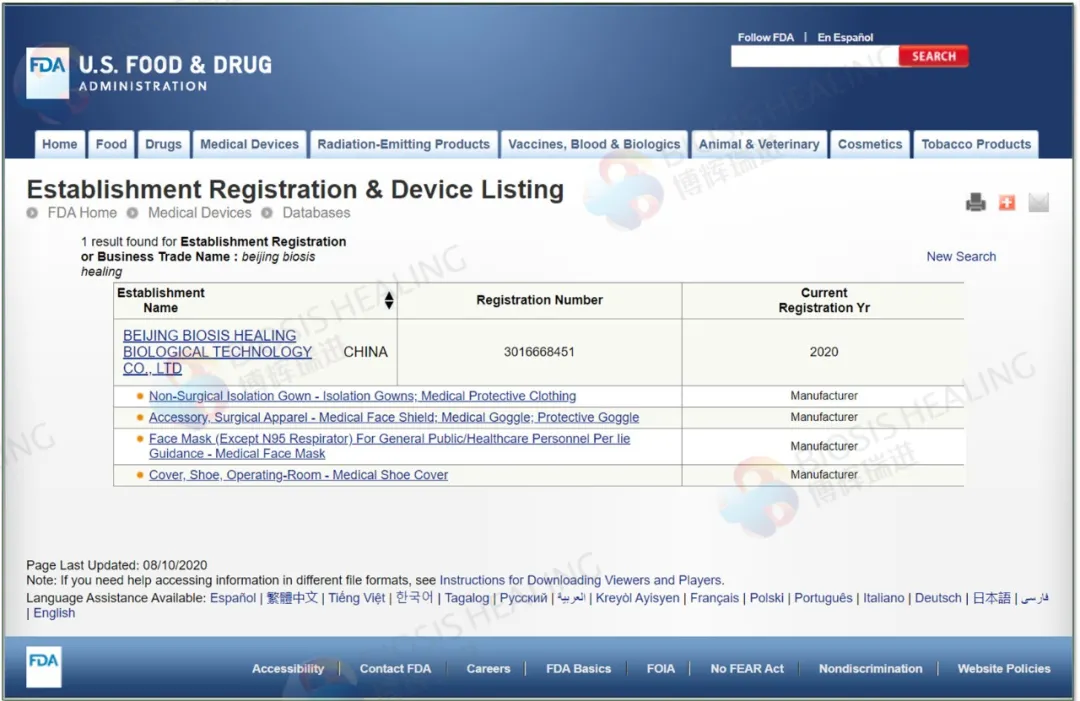
一次性医用防护服BH800-FDA
FDA Listing
中华人民共和国医疗器械注册证
China Certificate

无菌符合性声明DOC
Sterile DOC

非无菌符合性声明 DOC
Non-sterile DOC

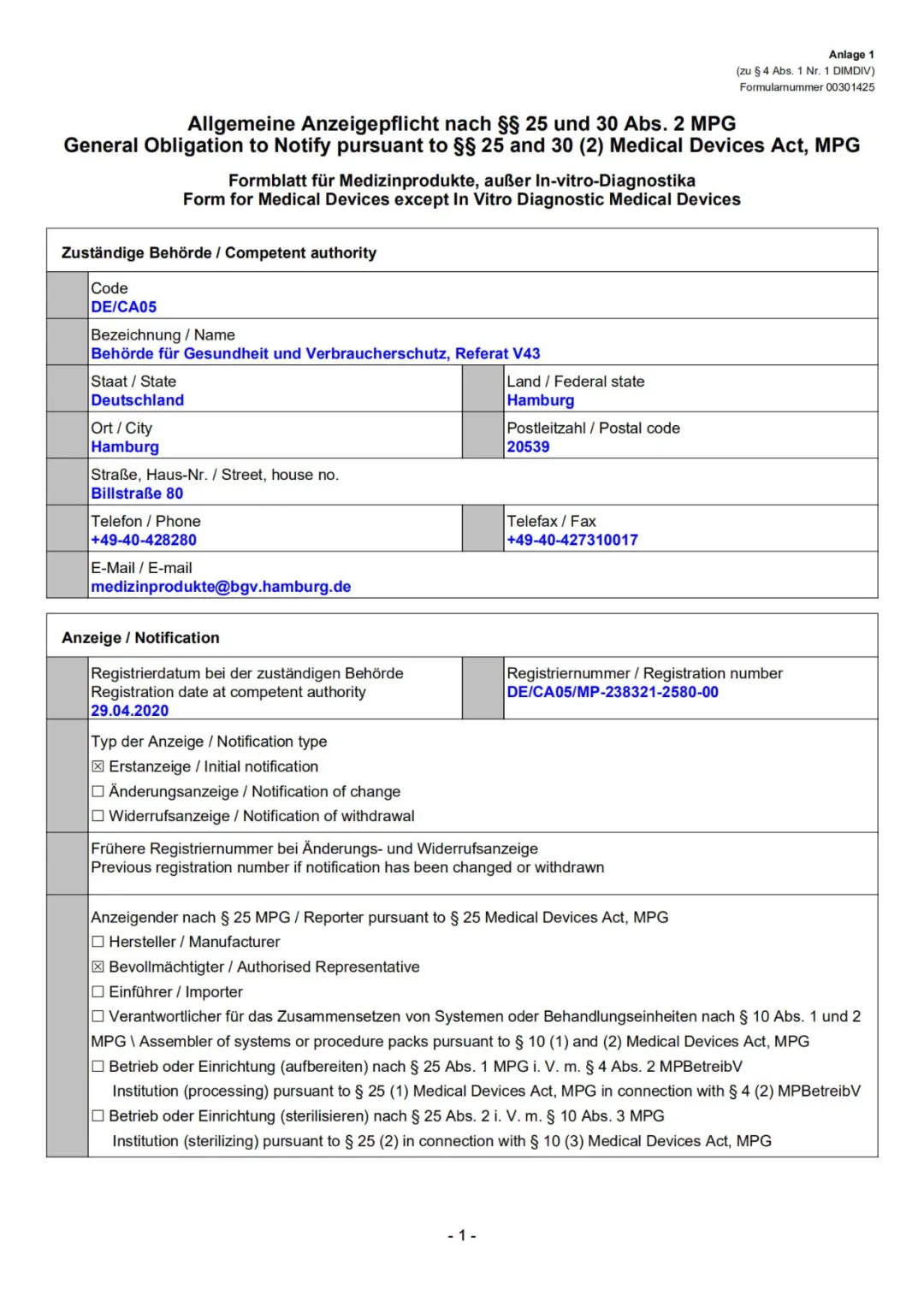
欧盟登记注册
EU Registration Certificate
EN 14126/Reach 检测报告
EN 14126/Reach Report

EN 14605 检测报告
EN 14605 Test Report
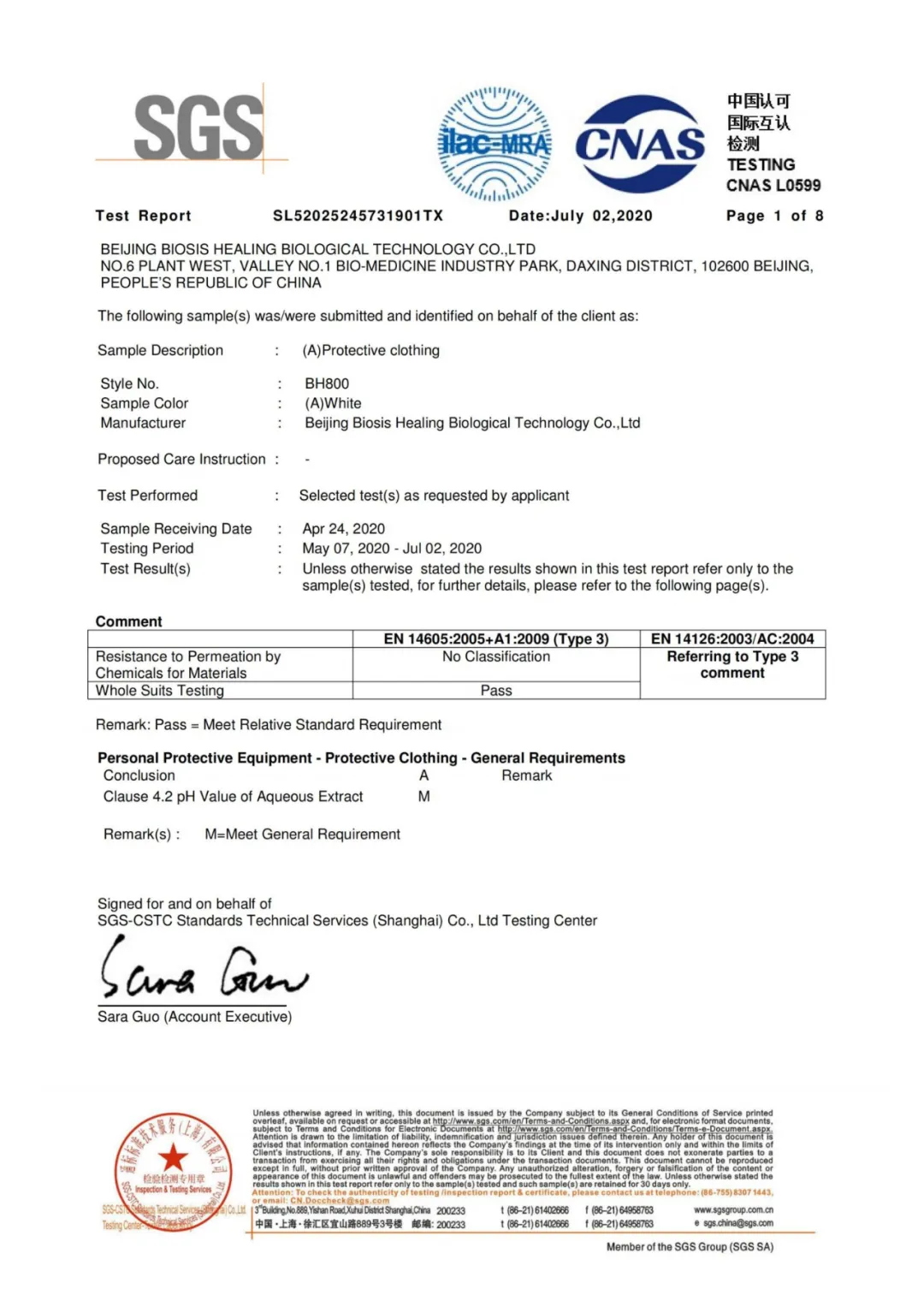
EN 14605 检测报告
EN 14605 Test Report
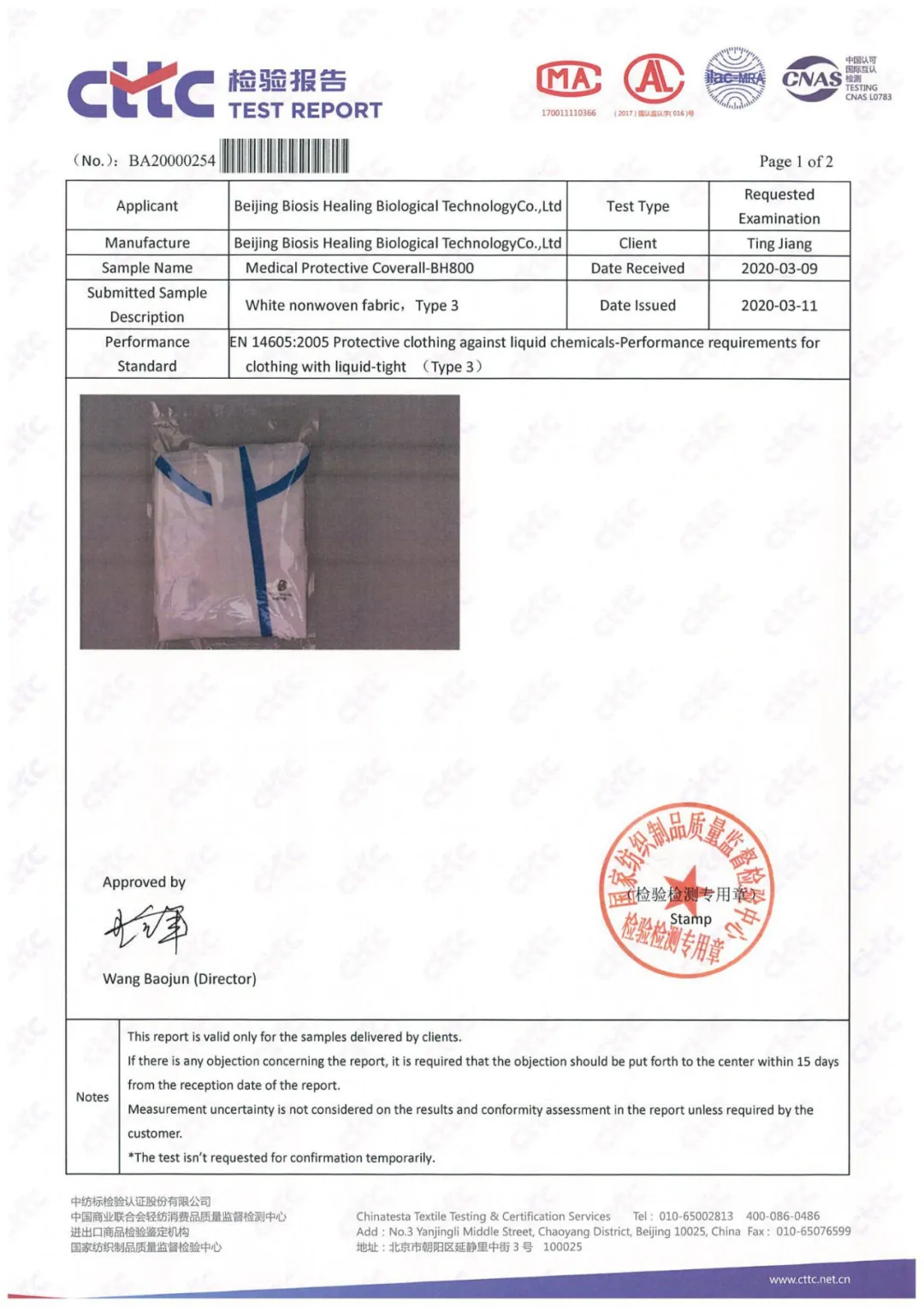
AAMI PB70 报告
AAMI PB70 Report

ISO 16604 报告
ISO 16604 Report

医疗器械出口销售证明
Export sales Certificate

企业海关出口资质
Customs qualification

医疗器械生产许可证
Production License


2024年12月6日,为期三天的SDHE...

理智弄槽外科韵,巴山蜀水天府情。2024...

6月11日,由中华口腔医学会主办的“口腔...

近日,《中华胸心血管外科杂志》2024年...

2024年5月9日,在VBEF未来医疗生...

新春伊始,万象更新。博辉瑞进迎来新春“开...

12月22日,《陕西省际联盟硬脑(脊)膜...

落叶飘零随风拂,初冬悄然而至。随着冬天一...

清风入梦时,春和正景明。四月芳菲至,等你...
对一个家庭来说,最大的喜事之一,莫过于添...
版权所有北京博辉瑞进生物科技有限公司 京ICP备16026579号 (京)-非经营性-2017-0030  京公网安备 11011502006176号 (京)网药械信息备字(2022)第00096号
京公网安备 11011502006176号 (京)网药械信息备字(2022)第00096号